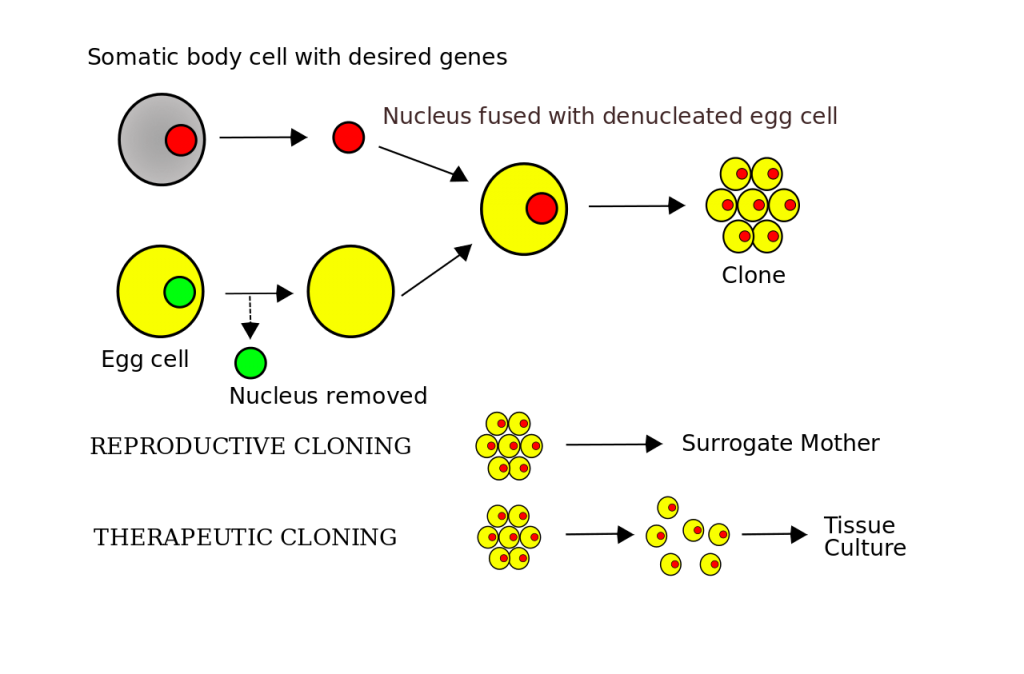
Just a few years before Dolly was born as the first surviving clone of a sheep in 1996, the movie Jurassic Park was launched, based on the same-named novel by Michael Crichton.[1,2] In this story scientists insert genetic material derived from fossils into amphibious eggs to bring all sorts of dinosaurs back to life. The actual cloning of animals follows a quite similar approach called somatic cell nuclear transfer or SCNT (fig 1): a nucleus with the desired DNA is isolated from a somatic (body) cell and introduced into an emptied ovum of the same species. Several electrical impulses excite the cell and stimulate proliferation in a nutritional medium. The most stable cell clusters, called blastomeres, can then be transferred to a host mother and grow into an embryo.[1] Dolly managed to fully develop into a lamb and lived 13 years until she died of an infection. She even gave birth to a lamb, proving the viability of cloned creatures.[3] Blastomeres that are dissected instead of implanted can be used to treat diseases or might enable the growth of tissue. Maybe in the future we will be even able to grow a whole surrogate organ ‒ an approach that is highly controversial since human somatic cells are mostly derived from embryotic tissue.[4]
According to a report from the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) about one million species of an estimated number of around 8 million species (only counting eukaryotes) on earth are currently endangered or threatened with loss of habitat.[6,7] In the history of Earth extinction has mostly been a consequence of natural disasters like climate change, volcanic eruptions, or meteorite impacts until human population started to expand.[8,9] The IPBES report demonstrates the present impact of human behaviour on biodiversity and it seems that we are facing many more extinctions caused by anthropogenic reasons in the next decades. It has become a growing interest to not only preserve existing species but also to revive those that have already died out.
One attempt is currently being made to revive Quaggas, a subspecies of the living plain zebra that has died out in the 1880s (fig 2), by selective breeding. Due to their close genetic relation some plain zebras that resemble the characteristic pattern of the quaggas have been selected in the hope to one day give birth to a zebra that looks just like them and shows similar genetic information.[10,11,12]

More demanding is the CRISPR Cas9 method: the DNA that can be extracted from most fossils like the woolly mammoth could be much too old to produce a healthy individuum. But their DNA might be partially recovered by replacing some sequences in the DNA of their closest living relative, the elephant, with extracted mammoth DNA. The genome will not be the same as it was millions of years ago and no one really knows how this will influence the livability of the animals.[13]
But most of the extinct species do not have such close relatives anymore. Interspecies nuclear transfer like in Jurassic Park can be another possibility for de-extinction, that means to revive species that have gone extinct or are on the verge of extinction. The San Diego Zoo Institute for Conservation Research maintains a large collection of cells and embryos called Frozen Zoo®.[14] By using reproductive technologies they develop methods to prevent endangered species like the northern white rhino or the Przewalski horse from extinction or inbreeding.[ 15] The first animal of an endangered species that was successfully cloned was a gaur (bos gaurus), an Asian ox, in 2001 by Advanced Cell Technology using genetic material from the San Diego Zoo. DNA from the skin cells of a male gaur were implanted into empty cow egg cells, grown into blastomeres that were then transferred into the wombs of domestic cows. One of eight embryos developed to a full-grown calf. Unfortunately, after being born, the gaur did not live for more than two days. However, the cause of death is considered to be an infection and not the fact that it is a trans-species clone.[16] The second clone that was created with the very same method had a higher life expectance. It was a banteng (bos javanicus), another endangered Asian cattle. Also remarkable is, that the used fibroblasts were taken and frozen 25 years before, in 1978.[17] An attempt to clone a species that has already gone extinct, the Pyrenean ibex (capra pyrenaica pyrenaica) failed since the kid was born with a deformed lung.[18]
The fact that cloned cells do in principle develop to embryos and even prolific adult animals (like Dolly) gives hope that one day species that have recently been wiped out could come back to life. But besides the challenging and time-consuming scientific research these plans also evoke a lot of critical questions in the society:
How is decided which species will be revived and which stays extinct?
It is clearly difficult to revive every species that we know has ever lived on this planet. There would just not be enough space and food and we might soon experience another wave of mass extinction. Since DNA from fossils might be too old, mammoths and dinosaurs are still out of question. This is shifting the focus on species of the recent past. But how can we select which species can live again and which won’t? We surely must consider the preservation of still existing species as a priority.
Where should they live?
If it is possible to clone many animals of one kind that can even mate, there must be a safe and nourishing environment, most likely captivity. Who knows how an entire species that has been created in captivity will develop? And the knowledge about the behaviour and needs of most of those animals is very little.[13]
Who is going to pay?
The scientist’s motivation might surely be an idealistic one but somehow all the research and maintenance must be financed. Innovations will always attract temporizers that try to exploit it financially. Zoos and wildlife parks that exhibit animals are the lesser problem. Some worry that wealthy poachers and “gourmets” who don’t withhold from hunting and eating endangered species now will just as much be attracted by the thought of getting hold of a cloned specimen. Paying to hunt an endangered species to support the protection financially is already practised in southern Africa and raises a lot of ethical issues.[19,20]
To see living “fossils” like dinosaurs, mammoths, dodos and all the others is surely an exciting thought. But if mankind proceeds like this, in just a few decades there might be much less animals on earth than there are now. Let’s hope that combined common sense, technical progress, and less vanity will lead to a preserved and healthy nature in our future.
‒Tatjana Dänzer
Read more:
[1] I. Wilmut, A. E. Schnieke, J. McWhir, A. J. Kind, K. H. S. Campbell, Nature 1997, 385, 810–813.
[2] M. Crichton, Jurassic Park, Alfred A. Knopf, Inc., 1990.
[3] http://www.roslin.ac.uk/publicInterest/DollyFinalIilness.php.
[4] S. Lü, Y. Li, S. Gao, S. Liu, H. Wang, W. He, J. Zhou, Z. Liu, Y. Zhang, Q. Lin, C. Duan, X. Yang, C. Wang, J. Cell. Mol. Med. 2010, 14, 2771‒2779.
[5] By en: converted to SVG by Belkorin, modified and translated by Wikibob – derived from image drawn by / de: Quelle: Zeichner: Schorschski / Dr. Jürgen Groth, with text translated, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=3080344.
[6] https://www.ipbes.net/news/Media-Release-Global-Assessment.
[7] C. Mora, D. P. Tittensor, S. Adl, A. g. B. Simpson, B. Worm, PLoS Biology, 2011, 9, 1‒8.
[8] D. B. Weishampel, P. Dodson, H. Osmólksa, The Dinosauria, 2nd ed., University of California, 2004.
[9] D. P. G. Bond, P. B. Wignall, Geological Society of America Special Papers, 2014, 505, 29–55.
[10] https://www.quaggaproject.org/.
[11] https://blog.nature.org/science/2014/10/13/quagga-can-an-extinct-animal-be-bred-back-into-existence/.
[12] J. A. Leonard, N. Rohland, S. Glaberman, R. C. Fleischer, A. Caccone, M. Hofreiter, Biol. Lett., 2005, 1, 291‒295.
[13] B. Shapiro, Genome Biology, 2015, 16, 1‒3.
[14] https://institute.sandiegozoo.org/resources/frozen-zoo%C2%AE, [15]https://institute.sandiegozoo.org/conservation-genetics.
[17] D. L. Janssen, A. L. Edwards, J. A. Koster, R. P. Lanza, O. A. Ryder, Reproduction, Fertility and Development, 2004, 16, 224‒224.
[18] https://faculty.mtsac.edu/cbriggs/Bringing%20them%20back%20to%20life%202013.pdf.
[19] http://www.bbc.com/future/story/20180328-the-increasingly-realistic-prospect-of-extinct-animal-zoos.
[20] https://www.pri.org/stories/2012-02-29/hunters-shoot-and-pay-save-rhino.