Tatjana Daenzer
Whenever scientists begin their experiments, a lot of time and brainpower has been invested in the preparation beforehand to ensure that everything goes right. Not only do they wish for positive results, meaning (in chemistry) high purity and high yield, but also a safe performance of their syntheses without unexpected events. Rigorous cleanliness and thorough research ensure success. But way too often the reaction vessels are not clean, or the necessary safety precautions are not met. Those who were so lucky not to set the lab on fire were sometimes even luckier to obtain something better than they were looking for. Daring curiosity is a human characteristic. Provokingly spoken, far before people conducted research as we know it today, chemistry – or alchemy – meant fooling around with whatever material was within reach, often without any factual knowledge to begin with. In fact, most of the basic materials on which we build our lives were the result of a little happy accident or just plain trial-and-error.
Glass occurs naturally in the form of some types of quartz, obsidian (an igneous rock that it is formed during volcanic eruptions) or tektites that were formed by meteoric impacts on the earth’s surface millions of years ago. It is a non-crystalline (amorphous) material, meaning that its molecular structure is not entirely ordered, resulting in transparency. Industrially it is obtained by smelting quartz sand, carbonates, and other trace minerals at temperatures around 1400 °C. It is not recorded who produced the first artificial glass. But archaeological findings suggest that it was already produced around the Eastern Mediterranean region of Western Asia between Egypt and Mesopotamia about 6000 years ago. According to a tale, “nitrum” (Latin for sodium hydrogen carbonate or natron) merchants were resting on a beach at the Syrian coast. Some nitrum lumps fell into the sand below their campfire, fusing to liquid glass.1 The temperature of a simple campfire, however, should not be able to melt sodium and sand (which is mostly consisting of silicates or quartz) this easily. Other sources report that the early glass was a byproduct of metal or pottery production.2 The heat that is needed to melt down ores is more sufficient to melt down silicates. Together with some impurities like salts and other minerals, that could have stuck to the ores, some grains of sand might have merged into some tiny beads of glass. The raw material was not that rare or expensive, but a lot of effort must have been put into finding out the right mixing ratio to gain clear, or even colorful glass. Thus, glass was a highly valued material for jewellery, comparable to actual gemstones. Nevertheless, both stories indicate that the discovery of glass production was a coincidence rather than a knowledge-based invention.
We don’t have to go too far back in history to find another phenomenon that was revealed by mistake but made a world-changing impact that we still can feel today: the discovery of vulcanization in 1839 by Charles Goodyear.
Rubber is a natural material that is harvested from plants. But in its raw form (also called latex), it has no desirable properties. On cold days it is brittle, on warm days it is a sticky liquid, and it stinks. There was, however, a commercial interest in a material that was elastic as well as weather- and waterproof, so Goodyear conducted experiments with latex and other substances to make the former more durable. Only by accident, he dropped a mixture containing latex and sulfur on a hot surface. Curiously, the mixture did not burn or evaporate, but formed a flexible goo that was stable at a convenient temperature range and could still be brought into a desired form. What the sulfur caused was “simply” a connection of the long chains of which the rubber is made of on the molecular level to form a cross-linked, flexible network. The method has been refined and still today new high-tech rubbers are developed.3,4
The serendipitous story of rubber goes even further. In search for a cheap substitute of rubber during WWII, Rob McGregor, Leathen Warrick and James Wright independently experimented with polysiloxane (silicon oil) and boric acid. The result was entirely nothing like the rubber that we use for tires and boots. But it was fun! They obtained an expandable soft mass that bounced when thrown on the hard ground but started to run after being left on a surface for some minutes. The material did not have any properties that were of industrial or military interest. But together with color, glitter, and even magnetic particles it later made a nice toy called “Silly Putty”.5,6

The decades that were overshadowed by the world wars were also inspired by a search for all sorts of cheap substitute materials. The first gas absorption refrigerators used liquefying gases like ammonia or sulfur dioxide as refrigerants. The leakage of these substances was certainly no fun. It did not take too long to discover that non-toxic, non-flammable and odorless refrigerants could be synthesized: the chlorofluorocarbons (CFCs). In 1938, occupied with the development of new CFCs, the chemist Roy J. Plunkett experimented with tetrafluoroethylene gas. When his gas cylinder suddenly clogged, he discovered, that the gas inside had been consumed to form a non-adherent, inert, and colorless solid: polytetrafluorethylene (PTFE). Apparently, the pressure together with the catalyzing effect of the metal of the cylinder wall led to a rapid polymerization of the gas. Although it was not suitable as a refrigerant, PTFE was quickly patented for its various beneficial properties, most of all its resistance to heat, the extremely low coefficient of friction (that means it is so slippery, that even geckos can’t hold a grip on a PTFE surface), and chemical stability.
PTFE went down in history, known under the trade name “Teflon” and still amazes cooks, plant manufacturers, and electricians all over the world.10-12
Again, it was a huge coincidence and a little bit of untidiness that led to another lucky (re)discovery, this time of a pharmaceutical treasure. Around the 1860s and 1870s, some research was conducted on the behavior of bacteria and fungi. Inspired by an episode with a patient who successfully treated a nasty ulcer with a broth of figs and milk, the German surgeon Theodor Billroth decided to experiment with bizarre broths as culture media for molds or fungi. He observed that a certain type of mold, the penicillium, prevented certain bacterial cultures (cocci) to grow in a medium. Around the same time, John Tyndall and Sir John Scott Burdon-Sanderson, both British amateur microbiologists, came to the same conclusion with their research. But it was not yet the time to deduce a correlation between infections, bacteria, and penicillin. The knowledge went by the board for decades because working with mingled microscopic cultures was not considered to be “true” science.14-16
The medical breakthrough had to wait for another roughly 50 years when Alexander Fleming, a Scottish physician, left some staphylococcus aureus cultures in a medium on his bench exposed to the air and went on holidays. When he came back, he noticed that one of his medium plates was “infected” with Penicillium glaucum which inhibited the bacteria that were too close to it to grow. It took some more years and some more brainpower to transform penicillin, as it was then called, to an ingestible drug for in vivo applications that was also producible in large quantities. But then, the triumphal march against otherwise painful or deadly infections like meningitis and syphilis was unstoppable.15,18-20
By the way, Fleming also discovered lysozyme (an antibacterial enzyme that plays an important part in the immune system) – by chance. For the discovery of penicillin, he and his co-workers received the Nobel Prize in Medicine in 1945.21

old advertisement for a teflon-coated pan from the 1960s.17
The color blue has fascinated mankind across all eras. For about 40% of all people, it seems to be the favorite color.23,24 Synthetic blue pigments based on minerals have already been known by ancient cultures like the Egyptians and the Chinese.25 Today, we still use Prussian blue (Iron(III) hexacyanoferrate(II)), cobalt blue (CoAl2O4), ultramarine (Na8 − 10Al6Si6O24S2 − 4) and indigo. Prussian blue was discovered in the early 18th century, when Johann Jacob Diesbach, a swiss pigment producer, attempted to make “Florentiner Lack”, a red varnish. The process involved the use of iron(II) sulfate. When potash was applied to clean up the product, the color of the batch unexpectedly turned deep blue. Diesbach asked his supplier Johann Konrad Dippel, and apparently, he accidentally delivered potash that was contaminated with animal fat and hexacyanoferrate, which did the actual trick. A little later, Johann Leonhard Frisch refined the synthesis and made it ready for the markets. Since he was from Berlin in the former state of Prussia, he named it “Prussian blue”.26
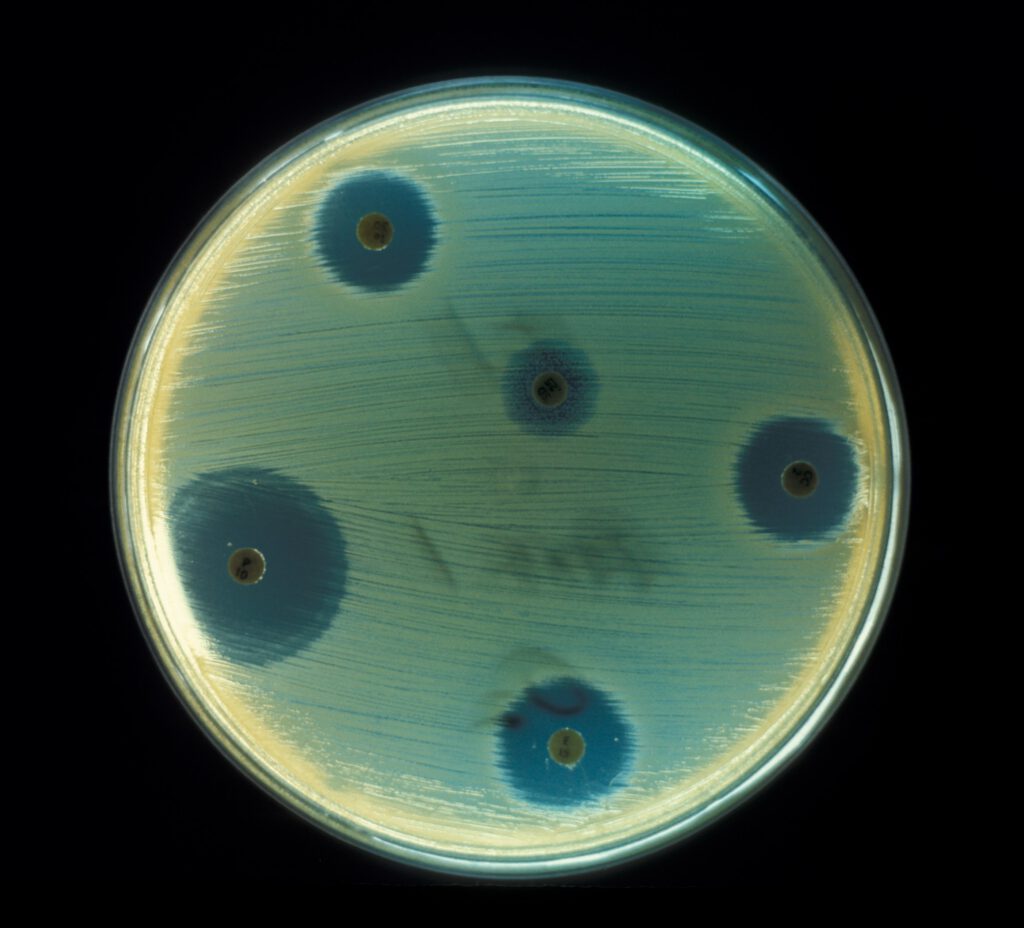
Antibiotics are dispersing from the discs of mold,
preventing the bacteria from growing around them.22
The student William Henry Perkin had similar luck in 1856 when he initially was looking for a way to synthesize quinine, a possible treatment for malaria. He attempted to oxidize aniline but the reagents which he used were contaminated with toluidine and the reaction resulted in a dark purple instead of a colorless precipitate. Mauveine was born and it was an excellent substitute for the expensive natural red dyes. Perkin patented his aniline-based dye and founded an own company at the age of only 18. In the end, this was the origin of some of today’s largest chemical companies, above all the “Badische Anilin- & Soda Fabrik”, BASF for short.27-29
The run on new brilliant synthetic pigments is not yet over and still relies on “happy accidents”. Andrew Smith was conducting his experiments in the lab of Prof. Mas Subramanian at the Ohio State University on the magnetic behavior of different manganese oxides and their solid-state mixtures. For their preparation high temperatures above 1000∘C are needed. When Andrew Smith pulled his baked samples out of the furnace, one of them was vividly blue. Upon further investigation the substance proved not only to be beautiful, but also chemically stable and non-toxic. The main components are yttrium, indium, and manganese (chemical formula: YIn1 − xMnxO3) and depending on their ratio, the darkness of the color can vary significantly. The pigment is already commercialized, and the company “Crayon” has given it a name that is more suitable for marketing: “Bluetiful”.30-32
This article can only give a short glimpse at the many happy coincidences that led to groundbreaking inventions. It is clearly an achievement of humankind not to throw unexpected and even undesired outcomes in the trash but to see some greater use and value in them. Hopefully, there will be many more of those accidents in science to further our relation to nature. Just imagine all the serendipities that still await us. Nevertheless, stay safe and curious!
Figure 5: The different shades of synthetic pigments and their respective rgb code in the brackets below.
Read more:
| [1] | G. Nightingale, 2020. Die zweifache “Erfindung” von Glas in der Bronzezeit und in der griechisch-römischen Antike. |
| [2] | J. Henderson, 2013. “Ancient Glass”. Cambridge University Press. pp. 127-157. |
| [3] | G. B. Kauffman, R. B. Seymour, J. Chem. Educ. 1990, 67, 422. |
| [4] | K.-D. Röker, “Vulkanisation – chemische Reaktion oder Adsorptionsvorgang? Eine Kontroverse zu Beginn des 20. Jahrhunderts.” Mitteilungen der Fachgruppe “Geschichte der Chemie” in der Gesellschaft Deutscher Chemiker, 2009, 20, 68. |
| [5] | M. Rob Roy, W. E. Leathen, “Treating dimethyl silicone polymer with boric oxide” (1943), Corning Inc., USA, US2431878A. |
| [6] | J. Wright, “Process for making puttylike elastic plastic, siloxane derivative composition containing zinc hydroxide” (1944), General Electric Co., USA, US2541851A. |
| [7] | Onohej zlatove – Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=63534845. |
| [8] | Hermann Junghans, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=27849835. |
| [9] | Lucasbosch – Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=23003624. |
| [10] | https://www.sciencehistory.org/historical-profile/roy-j-plunkett. |
| [11] | R. J Plunkett, “Tetrafluoroethylene polymers” (1939), Kinetic Chemicals Inc., USA, US2230654A. |
| [12] | G. J. Puts, P. Crouse, B. M. Ameduri, Chem. Rev., 2019, 119, 1763-1805. |
| [13] | Glitch010101 – Flickr, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1031643. |
| [14] | K. I. Mohr, History of Antibiotics Research. In: Stadler M., Dersch P. (eds) How to Overcome the Antibiotic Crisis. Current Topics in Microbiology and Immunology, 2016, 398. Springer, Cham. |
| [15] | G. Majno and I. Joris, Reviews of Infectious Diseases, 1979, 1, 880-884. |
| [16] | J. Tyndall, Phil. Trans. R. Soc., 1877, 167, 149-206. |
| [17] | trozzolo – Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=17229995. |
| [18] | E. Chain, H. W. Florey, M. B. Adelaide, A. D. Gardner, N. G. Heatley, M. A. Jennings, J. Orr-Ewing, J. A. G. Sanders, The Lancet 1940, 236, 226-228. |
| [19] | A. Fleming, The Lancet, 1943, 242, 434-438. |
| [20] | A. Fleming, Br. J. Exp. Pathol., 1929, 10, 226-236. |
| [21] | The Nobel Prize in Physiology or Medicine 1945. NobelPrize.org. Nobel Media AB 2021. Tue. 16 Mar 2021. https://www.nobelprize.org/prizes/medicine/1945/summary/. |
| [22] | CDC / Provider: Don Stalons – phil.cdc.gov, Public Domain, https://commons.wikimedia.org/w/index.php?curid=342353. |
| [23] | A. C. Hurlbert, Y. Ling, 2017, Colour Design: Theories and Applications, Janet Best, Editor. Woodhead Publishing, Elsevier, Duxford UK. 2nd Edition. pp 169-192. ISBN: 978-08-101270-3. |
| [24] | K. Lamancusa “Emotional Reactions to Color”. Creative Latitude. (https://web.archive.org/web/20160311005142/http://www.creativelatitude.com/articles/articles_lamacusa_color.html),retrieved 2021-03-07. |
| [25] | H. Berke, Chem. Soc. Rev., 2007, 36, 15-30. |
| [26] | A. Kraft, Bull. Hist. Chem., 2008, 33, 61-67. |
| [27] | K. Hübner, Chem. Unserer Zeit, 2006 40, 274-275. |
| [28] | M. M. Sousa, M. J. Melo, A. J. Parola, P. J. T. Morris, H. S. Rzepa, S. Seixas de Melo, Chem. Eur. J., 2008, 14, 8507-8513. |
| [29] | O. Meth-Cohn, M, Smith, J. Chem. Soc., Perkin Trans., 1994, 1, 5-7. |
| [30] | A. E. Smith, H. Mizoguchi, K. Delaney, N. A. Spaldin, A. W. Sleight, M. A. Subramanian, J. Am. Chem. Soc., 2009, 131, 47, 17084-17086. |
| [31] | https://today.oregonstate.edu/archives/2015/may/licensing-agreement-reached-brilliant-new-blue-pigment-discovered-happy-accident, retrieved 2021-03-07 |
| [32] | https://abcnews.go.com/Lifestyle/crayola-names-blue-crayon-bluetiful-retiring-yellow-dandelion/story?id=49825643, retrieved on 2021-03-07. |

