Curious things happen around us all the time – and sometimes we are so familiar with them that we do not even notice them anymore.
If you read the title you might now think that this article was about the Leidenfrost effect [1], that is, this little funny dance water droplets perform on a hot surface such as a frying pan. It is not, though. The Leidenfrost effect occurs when a material – usually a liquid – meets a surface far above its boiling temperature. A thin layer of the droplet’s surface will then evaporate rapidly, causing a protective gas coating to appear that effectively insulates the droplet and lets it last longer on the hot surface. Similar effects can also be seen with liquid nitrogen on a material at room temperature. These droplets appear to travel around due to ejected gasses. But does a similar phenomenon also occur without the necessity of a hot surface?
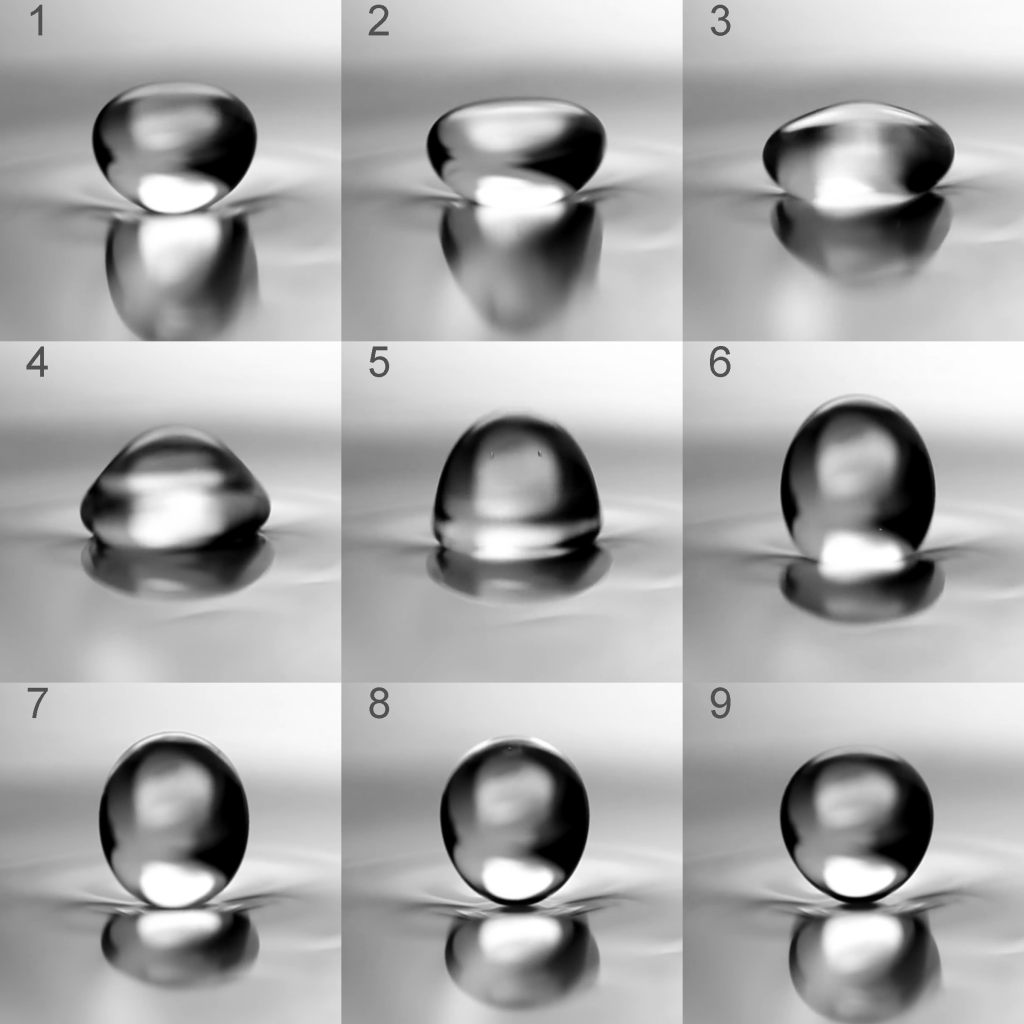
There is in fact a location where such an effect occurs regularly without us usually noticing: The bathroom. Under certain conditions water droplets can be seen moving on a surface of water as if they had hydrophobic properties. The easiest way to see them is in the shower, when the shower floor is already covered in a thin layer of water. If new water droplets now impact on this surface at certain angles and speeds, they can be seen rushing around for a while before disappearing. It turns out that in recent years a few scientific publications were dedicated to investigating this effect more closely. [2,3] With a high-speed camera, the bouncing effect can be visualized rather easily, as shown in Fig. 1: The droplet appears to cause a dent in the water surface and then bounce off without merging with the rest of the liquid. Of course, the first idea that comes into mind now is the Leidenfrost effect, where a similar behavior can be seen caused by a layer of vapor. However, here no high temperatures are involved and thus the generation of water vapor is negligible.
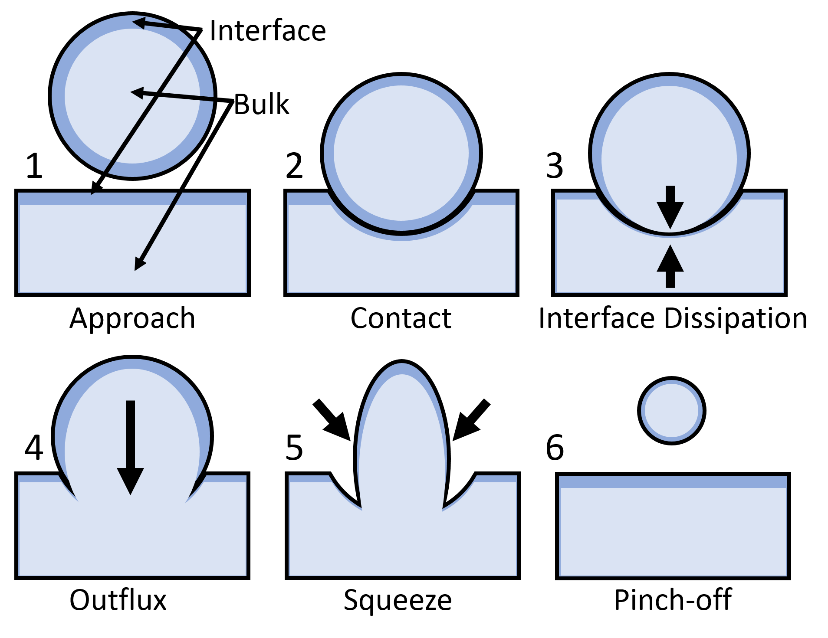
The first intuition of an air coating to protect the water droplet is still standing, though, and thus the scientists tried to model the behavior. It turns out that there is indeed a protective coating of air, which can get compressed when the droplet approaches the surface of the liquid underneath. The air simply cannot escape quickly enough and therefore protects the droplet on impact and pushes away from the water surface. This phenomenon causes what is called the residence time of a droplet, that is, the time a droplet can sit on top of a pool of the same liquid before coalescing (see Fig. 2). The theory was confirmed by lowering the ambient air pressure around the experiment, which caused the residence time to decrease. [4] However, one would expect that this thin layer of gas should not withstand a heavy impact of a droplet coming from e.g. the shower head with a lot of speed and thus kinetic energy.
An explanation can be found using a simple speaker membrane: When the droplets are put in contact with an oscillation surface, like water on an oscillating speaker, the bouncing is facilitated, and the droplets can remain intact for much longer. Moreover, the droplets now travel around just like they do in a shower! High-speed camera footage can show the reason for this change in behavior: The surface of the water pool, excited into periodic up- and down-movement patterns, gently catches the droplet if the surface is moving downwards in the moment of impact and therefore prevents the impact from destroying the protective gas layer. It is just like gently catching a water balloon with your hand by grabbing it in motion and then slowing it down. Additionally, the continuous movement of the surface seems to stabilize the gas layer and therefore massively increases the residence time, all while allowing the droplet to travel from minimum to minimum, thus creating the “walking water” effect. [6] In a shower, the impact of many, many droplets cause the surface of the water pool on the ground to oscillate in a similar manner, creating landing spots for some droplets that then move around the surface. The phenomenon can thus be explained by the residence time of a droplet together with an oscillating surface.
Finally, one can reproduce a similar behavior in space, where microgravity does not pull the droplets down. An air bubble inside of a water bubble can thus act like an isolated system where droplets can form and move… excited by the sound of a cello! If you got curious, please check out the beautiful footage in Ref. [6] where much of the inspiration of this article came from.
As stated initially, the most curious things happen around us and we simply have to notice them.
— Kai Litzius
References:
[1] https://www.engineersedge.com/physics/leidenfrost_effect_13089.htm
[2] Y. Couder et al., From Bouncing to Floating: Noncoalescence of Drops on a Fluid Bath, Phys. Rev. Lett. 94, 177801 (2005).
[3] J. Molácek & J. W. M. Bush, Drops bouncing on a vibrating bath, J. Fluid Mech. 727, 582-611 (2013).
[4] I. Klyuzhin et al., Persisting Water Droplets on Water Surfaces, J. Phys. Chem. B 114, 14020-14027 (2010).
[5] https://upload.wikimedia.org/wikipedia/commons/1/1d/Bouncing_droplets.gif
[6] https://www.youtube.com/watch?v=KJDEsAy9RyM (Water bubble in space at time index 8:18).