 While size is something easy to measure at the macroscopic scale it becomes a much more fuzzy concept as the size of the measured object gets smaller. At the molecular and subatomic level things get even worse as the objects of interest have now to be described by the wave-functions dictated by quantum mechanics.
While size is something easy to measure at the macroscopic scale it becomes a much more fuzzy concept as the size of the measured object gets smaller. At the molecular and subatomic level things get even worse as the objects of interest have now to be described by the wave-functions dictated by quantum mechanics.
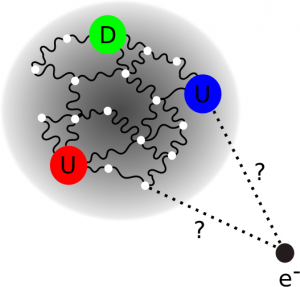
One way to still make sense of the dimension of an object is to imagine a simple geometric shape with the same physical properties (e.g. charge) of the object of interest. One then tries to find out how big this would have to be to reproduce the interaction with other particles that are observed in experiments. In this fashion one can think of the proton as a sphere with a certain distribution of charges and magnetic poles instead of the bubbling sea of quarks and gluons that it really is. The radius a charged sphere would have to correctly describe the Coulomb interaction between the proton and other particles is referred to as the charge radius of the proton.
To measure this one naturally needs particles that interact with the proton in this fashion. In principle one could do this with any charged particle but to make life easier one usually chooses electrons. This is done because an electron has no internal structure and can reasonably be thought of as a point particle. Therefore one does not need to take any fuzzyness in the geometry of the electron into account. Such interacting systems between protons and electrons can be achieved in two different ways. The first is to just shoot an electron at a proton and measure how much it is deflected. The second way is to let the electron orbit the proton and measure its energy. The first way is done in particle colliders all around the world. In the second way one deals with a hydrogen atom on which spectroscopy has to be performed. This is probably done in even more places.
To get more information about the proton from spectroscopy one can exchange the electron with its heavier cousin, the muon. In our current understanding the relevant difference between an electron and a muon is just their mass. A muon is much heavier than an electron and thus gets closer to the proton while orbiting it. As this increases the interaction between proton and muon the effect of the proton structure is much more visible in spectroscopy. The atom one ends up with in these experiments are called muonic Hydrogen.
Unfortunately the measurements of the proton charge radius from spectroscopy of muonic hydrogen [1] are significantly smaller than the ones obtained from proton-electron scattering [2].
Currently it is not clear why this is the case. Possible explanations range from problems in the data analysis or experimental uncertainty of fundamental constants to an insufficient theoretical description that gives the formulas from which the proton radius is calculated [3]. A more exotic explanation relies on so called “new physics”. This would mean our current understanding of the way fundamental particles interact is incomplete. If this is true it could be that the muon and the electron differ in their interaction with the proton so that they effectively see a particle of a different size [4]. Which of these will resolve the problem remains to be seen.
Stephan Koehler
[1] Antognini et al., “Proton Structurefrom Measurement of 2S-2P Transition Frequency of Muonic hydrogen”, Science 339, 417 (2013)
[2] Bernauer et al., “High-Precision Determination of the Electric and Magnetic Form Factors of the Proton”, Phys. Rev. Lett. 105, 242001 (2010)
[3] Barger et al., “Proton Size Anomaly”, Phys. Rev. Lett. 106, 153001 (2011)
[4] Carlson and Vanderhaegen, “Higher-order proton structure corrections to the lamb shift in muonic hydrogen”, Phys. Rev. A 84, 020102 (2011)