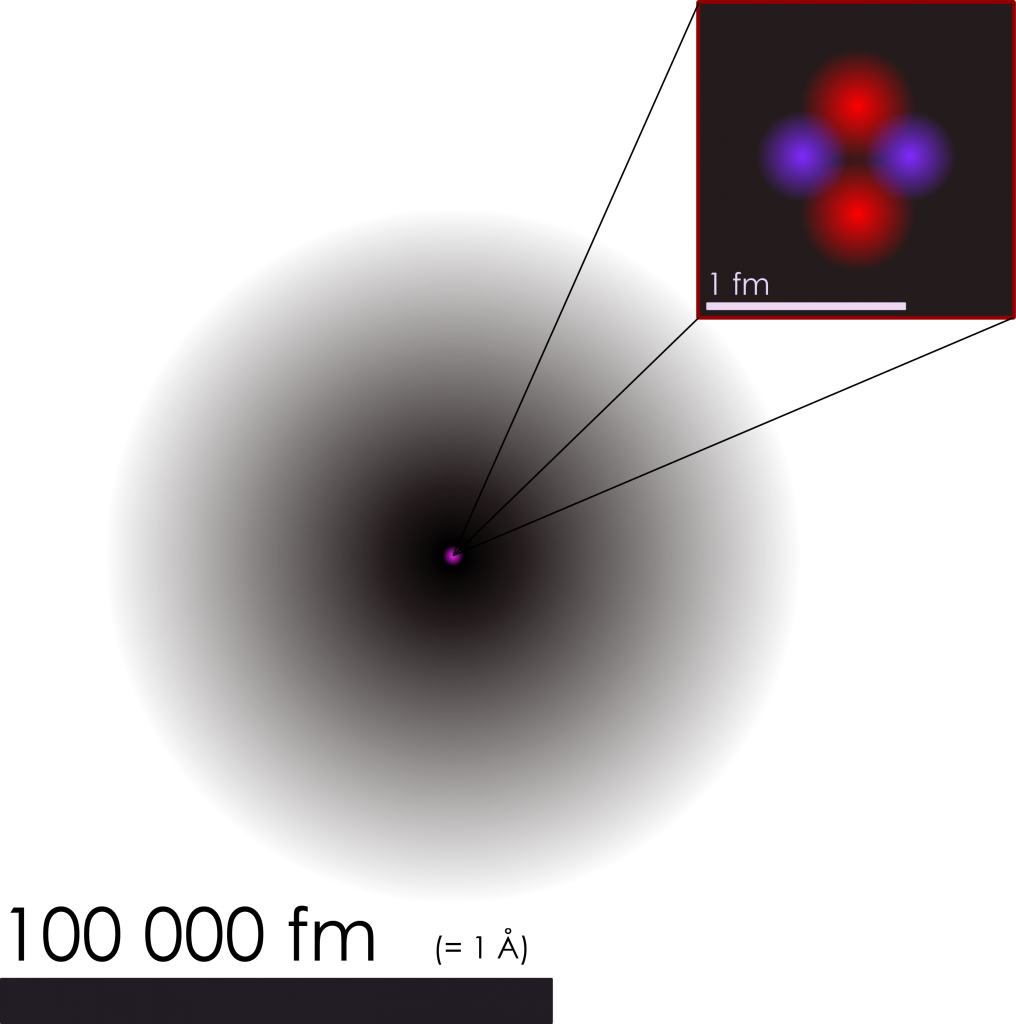
This might seem to be a very odd question at first, because we practically know everything about particles, atoms, molecules, and their sizes, right?
When we are in school, we learn that an atom is composed of a nucleus, which is very small in comparison to the atom itself and is surrounded by a “cloud of electrons”. This description implies already that we cannot be sure, where the electrons actually are; we describe this fact as electron densities, thus entailing that an atom does not have clearly defined edges. In theory, an electron can be found in any distance from the nucleus, but the probability decreases substantially when you go farther away. This is the case because of what we call wave-particle dualism.
Figure 1: Visualization of a Helium atom.(downloaded from https://upload.wikimedia.org/wikipedia/commons)
An electron behaves like a particle as we know from classical physics, but due to its very small size, an electron can also be described as a wave following the laws of quantum mechanics. Among other methods, people have used a type of scanning probe microscopy called atomic force microscopy (AFM) to determine actual radii of atoms. AFM relies on the detection of the interaction of a sample and a very sharp tip. It is a little bit as if a finger would profile an atomic surface. In contrast to optical microscopy methods, the resolution of AFM is not constrained by the optical diffraction limit, which makes the visualization of single atoms possible. But since this interaction between the tip and the sample atom depends on the respective electron clouds described by a certain wave function, it would not be fair to say that we know the definitive size of an atom.
– Kristina Klinker
Read more:
[1] F. J. Giessibl, Mater Today 2005, 8, 32–41.
[2] http://blog.thingswedontknow.com/2015/02/how-big-are-atoms.html (last access 10.01.16).