 Question of the Week, 25.5.2011
Question of the Week, 25.5.2011
It may be surprising that although water is so common and so well known to us from our everyday life scientists strongly disagree on how water molecules arrange themselves in the liquid phase. In the solid phase, when water freezes and becomes ice, the water molecules form a network that is held together by hydrogen bonds between the oxygen of one water molecule and the hydrogen atom of the adjacent water molecule. In ice every water molecule donates two hydrogen bonds and accepts to hydrogen bonds. But when the water is melted, about 10% of the hydrogen bonds are lost. The controverse question is what happens to these “lost” hydrogen bonds.
There are mainly two theories: The two-state theory claims that the water molecules go back and forth between two states, namely the ice-like structure and the broken hydrogen bond structure. Spokespeople of the second theory, the continuum theory, believe that there is a smooth transition between the ice-like and the broken hydrogen bond state.
Further reading:
http://www.lbl.gov/Science-Articles/Archive/sabl/2005/October/03-water-contoversy.html
http://www.chem1.com/acad/sci/aboutwater.html
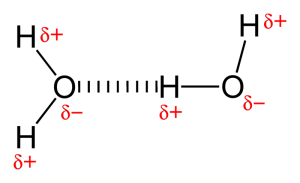
The picture shows two water molecules that are connected by one hydrogen bond.
Leonie Mueck