It is one of the most common educational experiments in school and straight from the books: The reaction of an alkali metal with water. During this reaction significant amounts of hydrogen gas are produced, which can ignite and thus explode due to the strongly exothermic reaction – at least that is the explanation one finds pretty much everywhere. However, there is something odd about this reasoning. On the one hand, a complete immersion of the metal within water should then prevent the explosion from happening as no oxygen is present to ignite the hydrogen gas. On the other hand, it is surprising that the solid-liquid interface of this heterogeneous reaction creates enough physical contact to drive the reaction. Additionally, the produced gas tends to separate the educts and therefore stop the reaction. Overall, there are quite a few unclear details in this proposed reaction mechanism.
A study of the Czech Academy of Sciences in Prague and the Technical University of Braunschweig, however, showed that even in presumably clear textbook reactions a lot of surprises may be found sometimes. [1,2] The scientists used drops of sodium-potassium alloy that is liquid at room temperature and filmed the reaction with high speed cameras. They could show that the explosive reaction also happens under water when the metal is completely immersed, thus ruling out the ignition of the hydrogen gas as the main driving mechanism for the explosion. Supported by molecular dynamics simulations, they instead showed what mechanism actually drives the reaction: A Coulomb explosion! During the reaction of a clean metal surface with the adjacent water molecules, electrons move quickly from the metal atoms into the water. This also explains why a solid piece of an alkali metal does not always explode in water: it needs a clean interface without significant oxidation. After the electrons left the metal surface and moved into the water, a strongly charged surface is left. On this surface, the ionized atoms strongly repel each other, and thus open up a path to more inner atoms that have not taken part in the reaction yet. On a time scale of about 0.1 ms, metal dendrites shoot into the water (see figure) and suddenly increase the surface area of the metal. [1-3] This happens extremely fast with giant charge currents flowing in the interface region. The surface tension is pretty much nullified in this case [2,3] and the expanding surface provides more reactive area. As a result, large amounts of hydrogen gas are suddenly produced. Together, these effects drive the explosion, while the ignition of the gas is not directly necessary for the explosion to occur. Instead, the hydrogen gas can also burn off later. [2]
Further results of the study could lead to approaches to avoid metal-water explosions and thus gain application relevance in industry. What is however most unusual about this study is that parts of it got funded by the YouTube science channel of the lead author of the paper, which he explicitly acknowledges. In this exciting case, science and media are really in a close relationship.
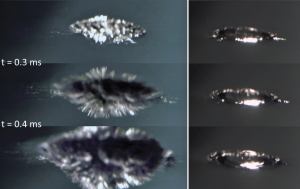
As soon as a drop of NaK-alloy gets in contact with water (top left), fine metal fingers are protruding into the water (middle). These are driven by the Coulomb explosion that massively increases the surface area and therefore the reactive interface. As a result, a fast production of hydrogen becomes possible, which further drives the explosion (bottom left). The right column depicts the impact of a water droplet for reference. [1,3]
— Kai Litzius
References:
[1] P. E. Mason et al., Nature Chemistry 7, 250–254 (2015).
[2] https://youtu.be/LmlAYnFF_s8
[3] https://youtu.be/xMfQSV4ygHE